https://www.nedo.go.jp/news/press/AA5_101710.
As part of the NEDO-subsidized “Strategic Energy Saving Technology Innovation Program” , Takasago Chemical Co., Ltd., Mitsubishi Tanabe Pharma Co., Ltd., Konica Minolta Chemical Co., Ltd., Yokogawa Solution Service Co., Ltd., and Tech Project Service Co., Ltd. Taisei Corporation, Shimadzu Corporation, Mitsubishi Kakoki Co., Ltd., and the National Institute of Advanced Industrial Science and Technology (AIST) have announced the development of a reconfigurable modular pharmaceutical manufacturing facility that uses a continuous production method. iFactory®, a demonstration plant was built at Takasago Chemical Kakegawa Factory, and a demonstration test was successfully conducted.
In the demonstration test, fully automatic continuous production at 10 kg scale was realised for more than 8 hours, and confirmed that the resulting compounds conformed to standards and had the same quality as batch production. Additionally, it was revealed that this method can reduce energy consumption by more than 80% and reduce waste by more than 60% compared to batch production, marking a major step toward on-demand production of pharmaceuticals.
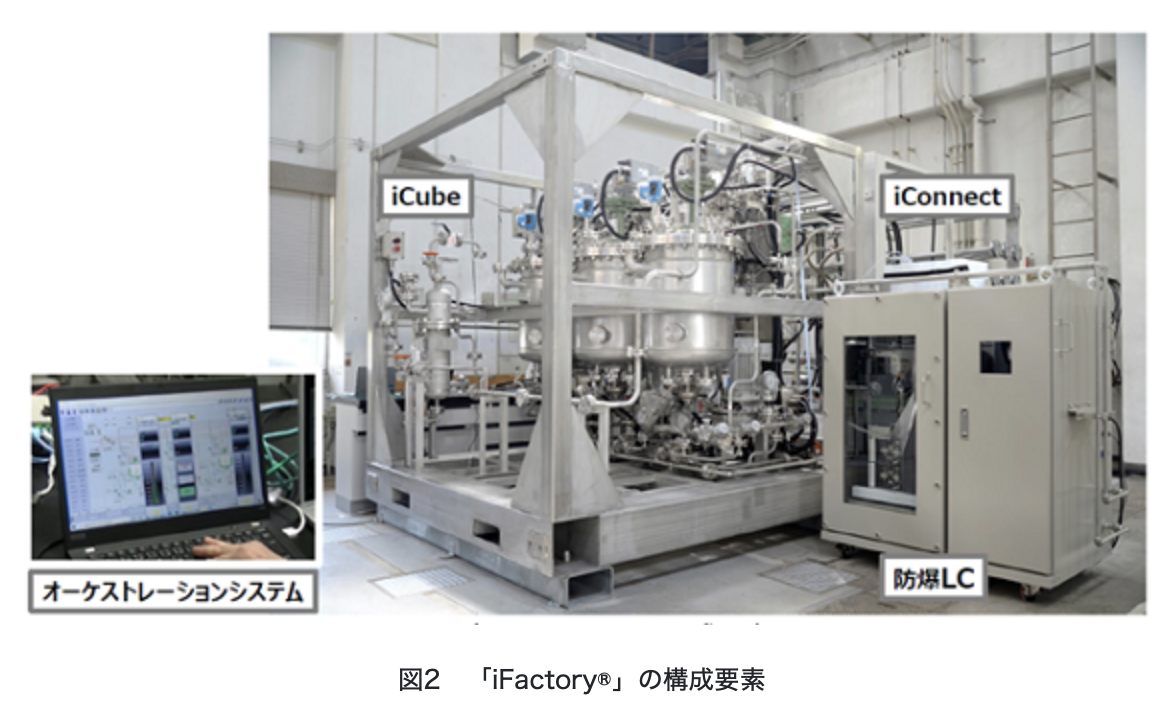
The manufacturing process is constructed by combining unit operations such as raw material supply, reaction, extraction, crystallization, filtration, drying, and filling. “iFactory®” has modularized these individual unit operations inside a cubic frame with a side of 2.3 m, and this modularized unit operation function is called “iCube.” Additional components of “iFactory®” are “iConnect,” a modular utility station that supplies utilities such as electricity and nitrogen to “iCube,” and “On-line’n On,” which performs quality analysis on site. It consists of an “explosion-proof ultra-high-performance liquid chromatograph system (explosion-proof LC)” and an “orchestration system” that automatically controls their interlocking. By interconnecting these, a series of manufacturing processes can be constructed, with the capacity to produce 10 kg of compounds per hour, and up to 72 tons per year by adjusting operating hours. Although it has a production capacity equivalent to a medium-sized batch production facility, the compactness of the facility allows it to save more than 50% of the space compared to a batch production facility.
In addition, a major advantage of iFactory® is that the manufacturing process can be reconfigured. With the demand for high-mix, variable-volume production, the advantage of being able to easily change the process and implement it in a short amount of time is huge, and to achieve this we have modularized unit operation functions. “iCube” is designed to weigh less than 5 tons and can be freely assembled and reconfigured using a forklift or crane. In addition, the software “orchestration system” that supports process reconfiguration allows processes to be reconfigured without reprogramming. This flexibility allows iFactory® to be easily applied to a variety of processes, and in the future allows for dual-use, where items can be quickly switched between normal times and emergencies such as pandemics, and equipment installed in other factories. It is also possible to quickly transfer technology to move production.