https://bio.nikkeibp.co.jp/atcl/news/p1/22/10/27/10073/
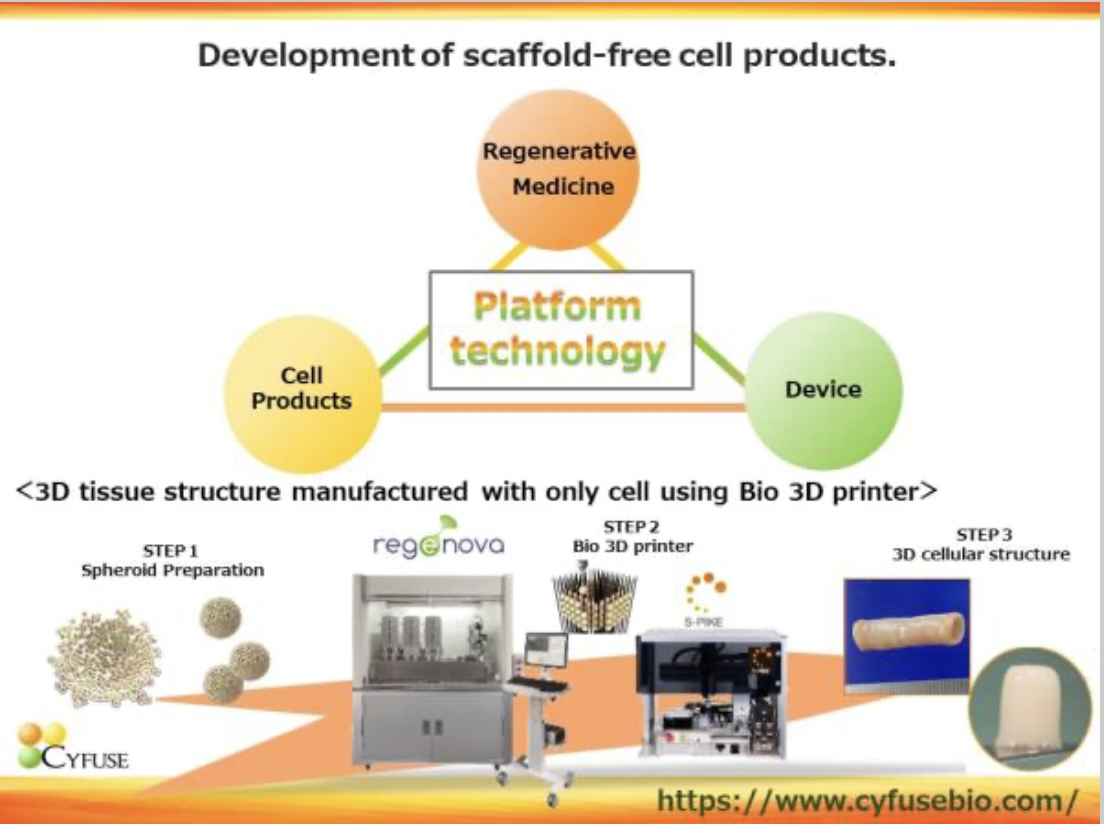
Cyfuse was established in 2010 and is focused on 3D cell technology that uses a bio 3D printer to laminate cell clumps (spheroids) consisting of an average of 10,000 cells in a 3D manner without the use of artificial scaffolds. The company has 3 business fields:
- to develop a pipeline of regenerative medicine products and cell products for research on consignment;
- to develop cell products as tools to support drug discovery for pharmaceutical companies; and
- to develop bio 3D printers and consumables.
Currently, there are three products in the pipeline for development in the regenerative medicine field.
- a regenerative medicine product candidate (development number: CYF-PA1) that uses fibroblasts harvested from patients and stacked into a tube approximately 3 cm in length for the regeneration of peripheral nerves. Currently, physician-led clinical trials for CYF-PA1 are being conducted for peripheral nerve injury patients, and the company plans to start company clinical trials in 2023.
- a regenerative medicine product candidate (development number: CYF-CA1), in which stem cells are isolated from fat harvested from patients’ buttocks for bone and cartilage regeneration, and the adipose-derived stem cells (MSCs) are stacked in a cylindrical shape. So far, a clinical study has been conducted for patients with osteochondritis dissecans and other conditions, safety has been confirmed and proof of concept (POC) has been obtained. Currently, preparations are underway for investigator-initiated clinical trials, which will begin in 2023, and the company plans to start corporate clinical trials in 2024.
- a candidate for a regenerative medicine product (development code: CYF-BA1), which consists of fibroblasts collected from patients and stacked in a tube shape of about 5 cm in order to regenerate blood vessels. Clinical research on CYF-BA1 is currently underway at Saga University Hospital for end-stage renal failure patients who are undergoing hemodialysis and experiencing problems such as stenosis or blockage in the shunt. The company plans to start clinical trials in 2023 or later, file for approval in 2027, and obtain approval by the end of 2027.
Cyfuse raises about 13 million € for 3D printed organoids