https://sj.jst.go.jp/news/202601/n0109-03k.html
https://www.nature.com/articles/s41598-025-88616-x
https://www.sanyo-chemical.co.jp/wp/wp-content/uploads/2025/10/k20251031.pdf
Knee osteoarthritis is caused by damage to the knee joint cartilage and meniscus that occurs with aging or sports. Meniscus injuries are treated with either repair surgery or resection surgery in which the meniscus itself is removed. While meniscus injuries are common in elderly people, they also frequently occur in younger people due to sports-related trauma.
The meniscus consists of two regions: the outer and inner. The success of repair surgery depends on blood flow, and surgery is not applicable to injuries in the inner avascular zone.
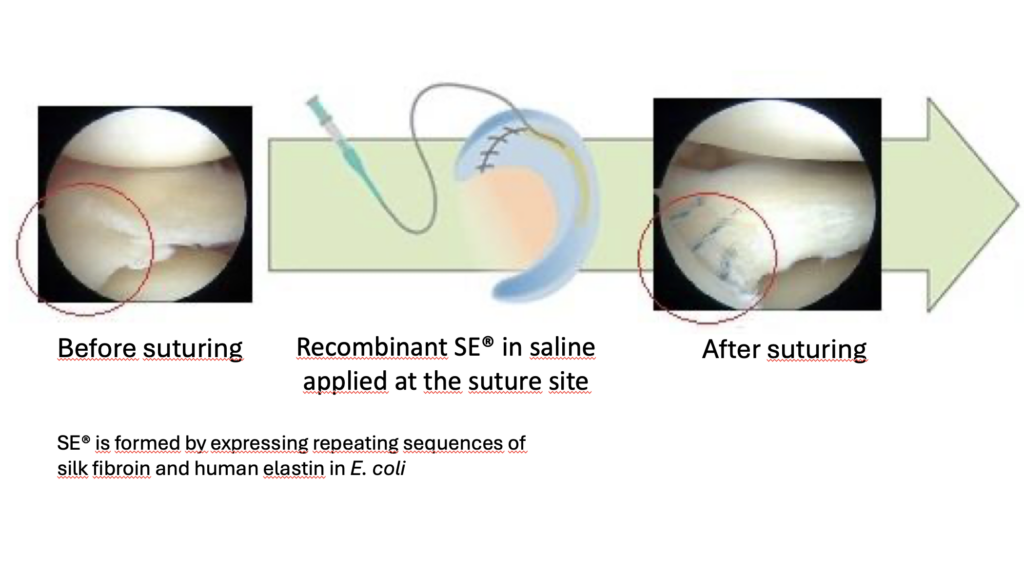
Through collaborative research with researchers at Hiroshima University, Sanyo Chemical discovered the potential application of Silk Elastin®, a recombinant functional protein produced in E. coli, promotes tissue repair and regeneration and can be used for the treatment of meniscus injuries. An investigator-initiated clinical trial with 8 patients using this material conducted at the hospital yielded favorable safety results: meniscus preservation through healing was successful in six of the eight cases, with good results obtained even in cases that were difficult to treat with conventional methods. The results were published in Nature.
Building on these results, a corporate clinical trial will be conducted at multiple facilities across Japan, including Hiroshima University Hospital, with the aim of collecting more extensive data and verifying efficacy and safety in detail. In this clinical trial, repair surgery is performed arthroscopically, and a SE aqueous solution is injected and put in place. The SE transforms into a gel that serves as a scaffold for cell proliferation, helping the meniscus to heal.
SE is a recombinant protein biosynthesized by introducing repeating sequences of silk fibroin and human elastin into Escherichia coli. It has already received regulatory approval as a wound healing material for refractory skin ulcers and is scheduled to begin sales in 2026.
The SE obtained through extraction and purification has a cotton-like texture. Previous basic research has confirmed features such as activation of cell migration and enhanced collagen production, and its high safety has been verified since it is not derived from animals.
I