https://www.nature.com/articles/s43856-025-00959-8
https://sj.jst.go.jp/news/202508/n0825-03k.html
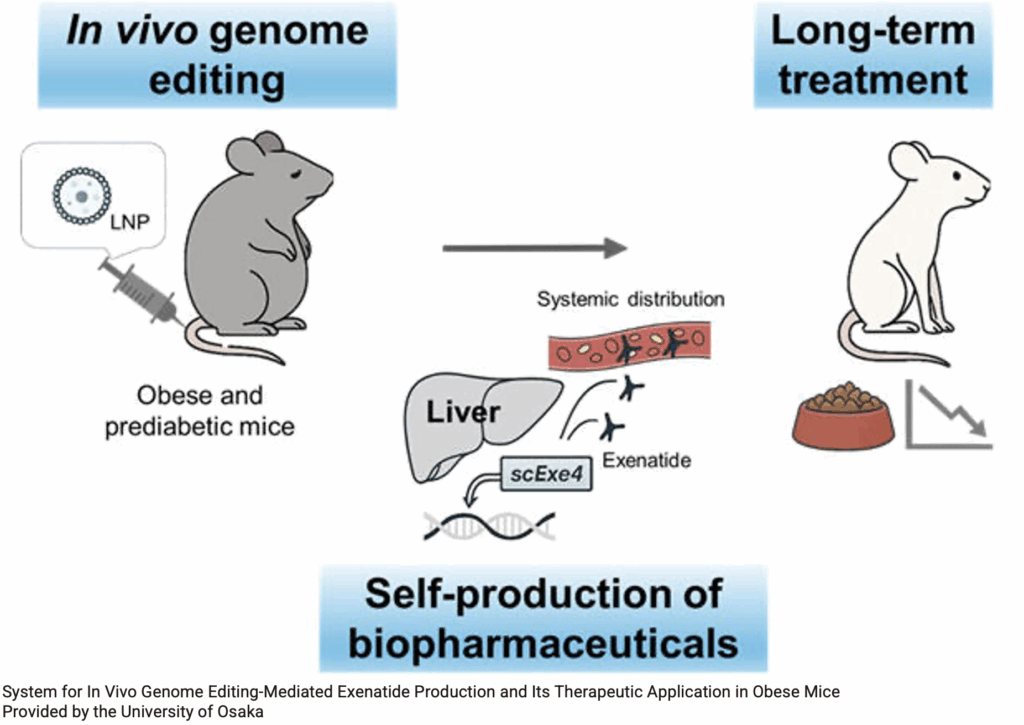
Exenatide, a GLP1 receptor agonist, is known to have high efficacy in suppressing appetite and reducing weight, but requires regular injections, and weight returns to baseline when treatment is discontinued.
A research group at Osaka University has used obese mice and expressed exenatide in their liver, as an in-body factory. They developed a genome editing strategy to integrate an exenatide-expressing gene construct into liver cells in the body. based on the albumin-producing gene, which is constantly produced in large quantities in liver cells. In order to deliver this gene construct to the liver, they administered the genes in lipid nanoparticles (LNPs).
When this genome editing LNP was administered just once by injection to mouse models of diet-induced obesity and pre-diabetes, exenatide was detected in the mouse blood for over 6 months. This indicates that liver cells were continuously producing exenatide thanks to genome editing. Furthermore, they found that two administrations resulted in twice the amount of exenatide being secreted long-term compared to single administration, suggesting the possibility of adjusting treatment effects.
Obese mice that received this genome editing treatment had suppressed appetite and were significantly prevented from gaining weight. The researchers also confirmed improvement in abnormal blood glucose levels, a symptom of pre-diabetes, and enhanced insulin sensitivity (improved insulin responsiveness). These effects were at the same level as healthy mice fed a normal diet, demonstrating that this genome editing treatment is highly effective against diet-induced obesity and pre-diabetes. They also confirmed that exenatide produced in the body did not adversely affect the secretion of GLP-1, a hormone naturally present in the body. Regarding safety, no off-target effects causing unintended changes to other genes were observed, and blood markers indicating liver function were normal, demonstrating no major safety concerns.