https://pubs.acs.org/doi/10.1021/acscatal.4c00487
Sortases are prokaryotic enzymes that modify surface proteins by recognizing and cleaving a carboxyl-terminal sorting signal.
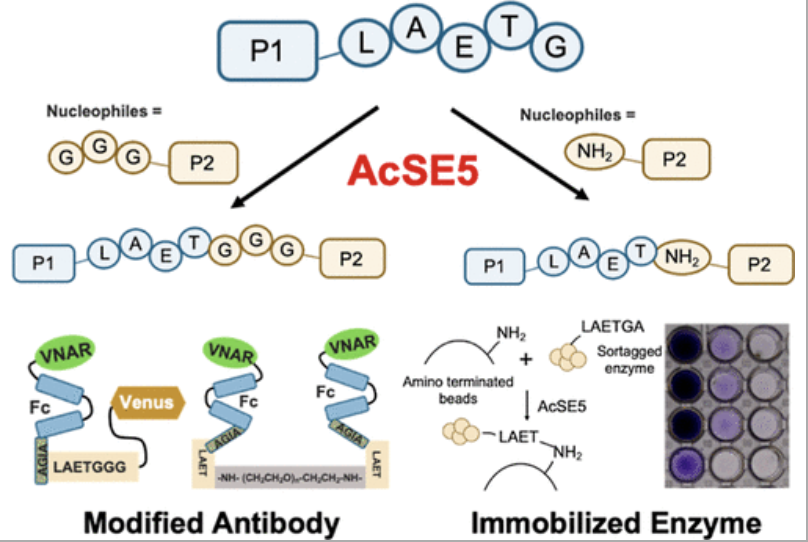
A team of Japanese scientists has reconstructed an ancestral sortase E, named AcSE5, through a combination of sequence data mining and ancestral sequence reconstruction. AcSE5 is a Ca2+ independent sortase and recognizes donors with LAETG at their C-termini. It can employ peptides bearing GGG- or GAA-at N-terminus and straight-chained alkylamines as nucleophiles. Using this enzyme, two protein conjugates were synthesis:
- a variable new antigen receptor and dual-conjugated variable new antigen receptor via poly(ethylene) glycol diamine
- an amino acid oxidase immobilized to amino-terminated polystyrene beads
Peptide conjugation exceeded 70% under optimal conditions, and protein functions were maintained.