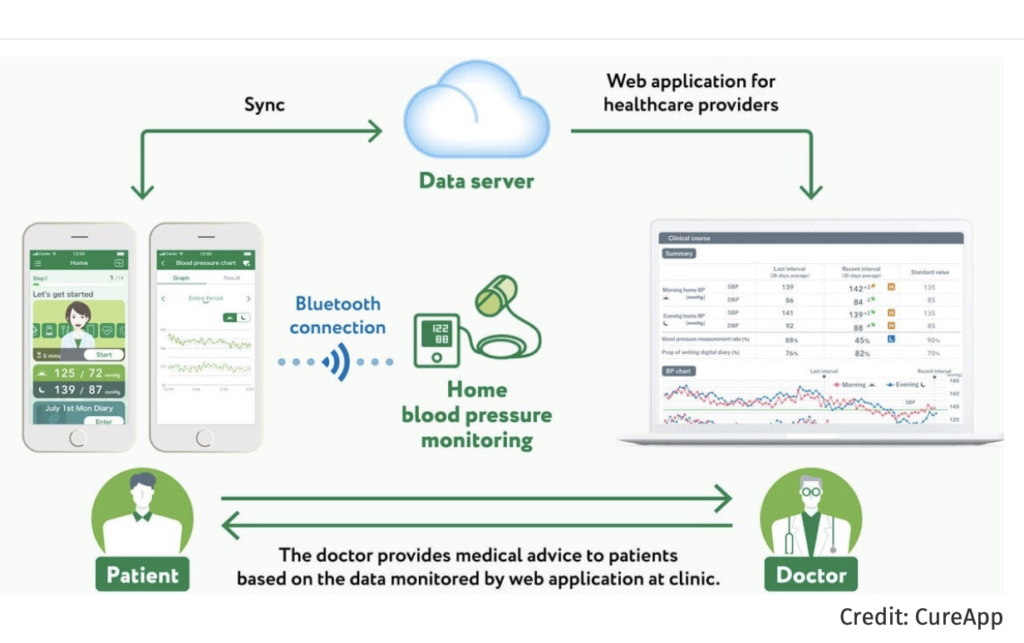
CureApp, a startup in Tokyo, is expected to obtain approval this April for its non-pharmacological digital therapy (DTx) of hypertension (rather: support of therapy). The medical device consists of two components: 1. an app to be used by the patient, and 2. an app for the physician to review the patient’s data. The patient enters daily blood pressure, diet, and other lifestyle information through the smartphone. Based on this information, an algorithm in the app selects appropriate advice to encourage the patient to change his/her behavior, and displays it in the app. Doctors can use the app to check the patient’s blood pressure and lifestyle record to help them in their medical treatment.
Before application, CureApp had conducted a clinical trial based on 24-hour blood pressure monitoring (ABPM). It was concluded that use of the app led to a 10% reduction in the risk of vascular events.
Nikkei Biotech news release, March 11, 2022