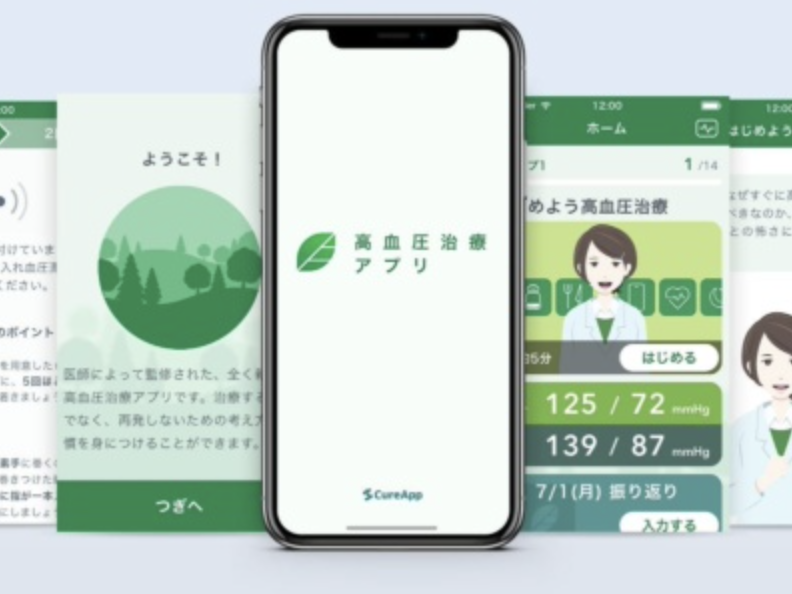
On March 9, 2022, the Ministry of Health, Labor, and Welfare approved CureApp’s HT hypertension control, the first software digital therapy (DTx) in Japan. Approval is expected for April 2022 and is
The app is meant to assist in non-pharmacological therapy for patients with essential hypertension. It consists of two components: (1) an app for patients to use and (2) an app for doctors to check patient data. The process of using the app is as follows. First, the patient enters daily blood pressure, diet, and other lifestyle information via the smartphone app. Based on this information, an algorithm in the app selects appropriate advice to promote behavioral changes in the patient, which is then displayed in the app. Doctors can use the app to check the patient’s blood pressure and lifestyle record to help them in their medical care.
CureApp conducted a clinical trial from January to December 2020 to apply for approval of the application for the treatment of hypertension. The primary endpoint was the change from baseline in mean systolic blood pressure (SBP) over a 24-hour period as measured by 24-hour blood pressure monitoring (ABPM). The results of the trial showed that SBP in the intervention group compared to the control group was -2.4 mmHg. MHLW officials explained, “The clinical significance of this result was also discussed, and based on the data submitted by CureApp and discussions with experts, we concluded that it corresponds to a 10% reduction in the risk of vascular events.